Peripheral Portfolio:
Diamondback 360® Peripheral Orbital Atherectomy System
Diamondback 360® Peripheral Orbital Atherectomy System – Exchangeable Series
Zilient® Peripheral Guidewires
ViperCath™ XC Peripheral Exchange Catheter
Stealth 360® Peripheral Orbital Atherectomy System
ViperWire Advance® and ViperWire Advance® with Flex Tip Peripheral Guide Wires
Sapphire® II PRO Balloon Dilatation Catheter
Teleport® Microcatheter
WIRION® Peripheral Embolic Protection System
JADE® PTA Balloon Catheter
ViperCross™ Support Catheter
Shepherd™Peripheral Guidewire
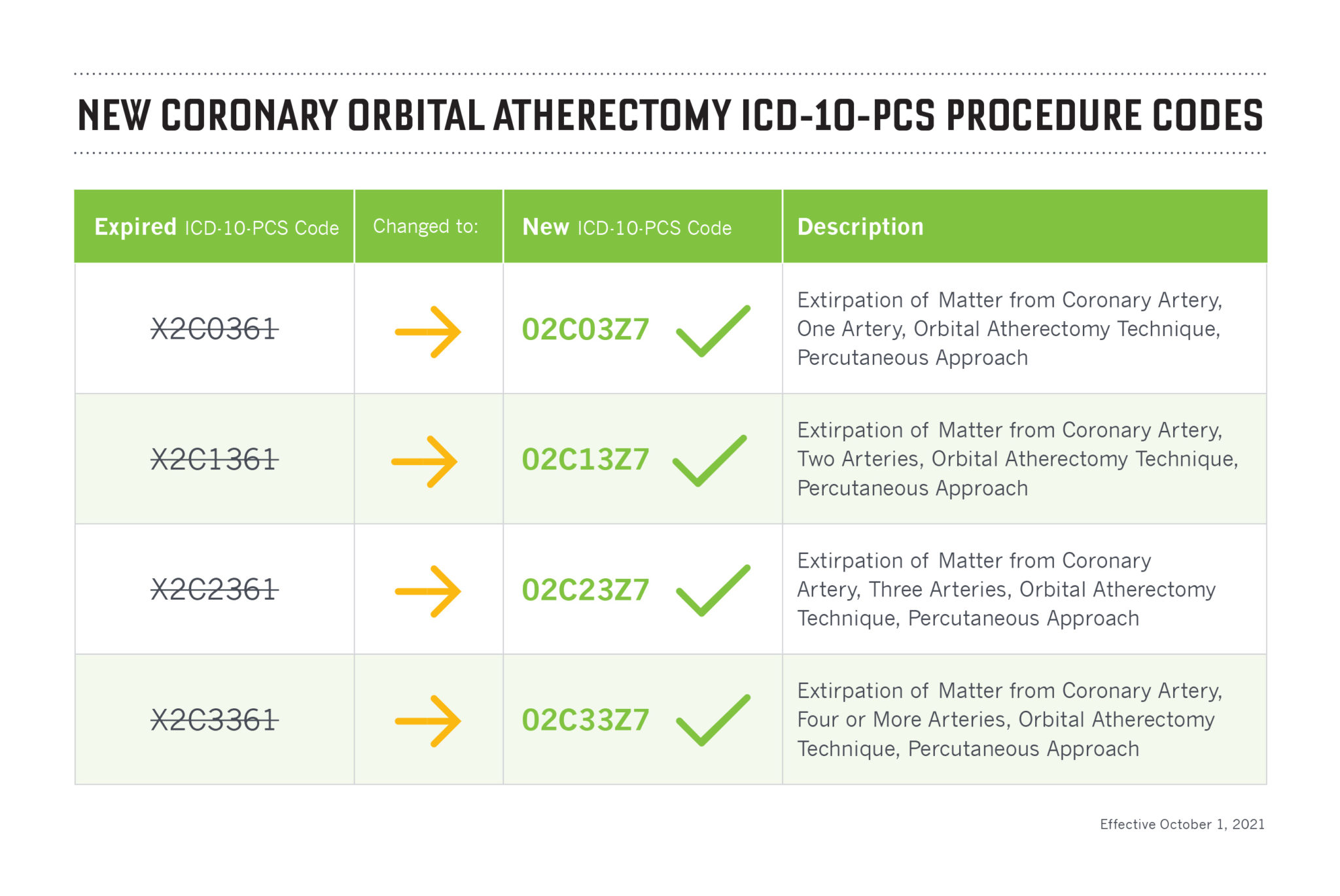
Diamondback 360® Coronary Orbital Atherectomy System
Indications Statement: The Diamondback 360® Coronary Orbital Atherectomy System (OAS) is a percutaneous orbital atherectomy system indicated to facilitate stent delivery in patients with coronary artery disease (CAD) who are acceptable candidates for PTCA or stenting due to de novo, severely calcified coronary artery lesions.
Contraindications: The OAS is contraindicated when the ViperWire Advance® Coronary Guide Wire cannot pass across the coronary lesion or the target lesion is within a bypass graft or stent. The OAS is contraindicated when the patient is not an appropriate candidate for bypass surgery, angioplasty, or atherectomy therapy, or has angiographic evidence of thrombus, or has only one open vessel, or has angiographic evidence of significant dissection at the treatment site and for women who are pregnant or children.
Warnings/Precautions: Performing treatment in excessively tortuous vessels or bifurcations may result in vessel damage; The OAS was only evaluated in severely calcified lesions, A temporary pacing lead may be necessary when treating lesions in the right coronary and circumflex arteries; On-site surgical back-up should be included as a clinical consideration; Use in patients with an ejection fraction (EF) of less than 25% has not been evaluated.
See the instructions for use before performing Diamondback 360 Coronary Orbital Atherectomy System procedures for detailed information regarding the procedure, indications, contraindications, warnings, precautions, and potential adverse events.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
ViperWire Advance® and ViperWire Advance® with Flex Tip Coronary Guide Wires
Indications Statement: The Diamondback 360® Coronary Orbital Atherectomy System (OAS) is a percutaneous orbital atherectomy system indicated to facilitate stent delivery in patients with coronary artery disease (CAD) who are acceptable candidates for PTCA or stenting due to de novo, severely calcified coronary artery lesions.
Contraindications: The OAS is contraindicated when the ViperWire Advance® Coronary Guide Wire cannot pass across the coronary lesion or the target lesion is within a bypass graft or stent. The OAS is contraindicated when the patient is not an appropriate candidate for bypass surgery, angioplasty, or atherectomy therapy, or has angiographic evidence of thrombus, or has only one open vessel, or has angiographic evidence of significant dissection at the treatment site and for women who are pregnant or children.
Warnings/Precautions: Performing treatment in excessively tortuous vessels or bifurcations may result in vessel damage; The OAS was only evaluated in severely calcified lesions, A temporary pacing lead may be necessary when treating lesions in the right coronary and circumflex arteries; On-site surgical back-up should be included as a clinical consideration; Use in patients with an ejection fraction (EF) of less than 25% has not been evaluated.
See the instructions for use before performing Diamondback 360 Coronary Orbital Atherectomy System procedures for detailed information regarding the procedure, indications, contraindications, warnings, precautions, and potential adverse events.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
Diamondback 360® Peripheral Orbital Atherectomy System
Indications Statement: The Diamondback 360® Peripheral Orbital Atherectomy System is a percutaneous orbital atherectomy system indicated for use as therapy in patients with occlusive atherosclerotic disease in peripheral arteries and who are acceptable candidates for percutaneous transluminal atherectomy. The OAS supports removal of stenotic material from artificial arteriovenous dialysis fistulae (AV shunt). The system is a percutaneous orbital atherectomy system indicated as a therapy in patients with occluded hemodialysis grafts who are acceptable candidates for percutaneous transluminal angioplasty.
Contraindications: The Systems are contraindicated for use in coronary arteries, bypass grafts, stents, or where thrombus or dissections are present.
Adverse Events: Although the incidence of adverse events is rare, potential events that can occur with atherectomy include: pain, hypotension, CVA/TIA, death, dissection, perforation, distal embolization, thrombus formation, hematuria, abrupt or acute vessel closure, or arterial spasm.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
Diamondback 360® Peripheral Orbital Atherectomy System – Exchangeable Series
Indications Statement: The Diamondback 360® Peripheral Orbital Atherectomy System – Exchangeable Series is a percutaneous orbital atherectomy system indicated for use as therapy in patients with occlusive atherosclerotic disease in peripheral arteries and who are acceptable candidates for percutaneous transluminal atherectomy. The OAS supports removal of stenotic material from artificial arteriovenous dialysis fistulae (AV shunt). The system is a percutaneous orbital atherectomy system indicated as a therapy in patients with occluded hemodialysis grafts who are acceptable candidates for percutaneous transluminal angioplasty.
Contraindications: The Systems are contraindicated for use in coronary arteries, bypass grafts, stents, or where thrombus or dissections are present.
Adverse Events: Although the incidence of adverse events is rare, potential events that can occur with atherectomy include: pain, hypotension, CVA/TIA, death, dissection, perforation, distal embolization, thrombus formation, hematuria, abrupt or acute vessel closure, or arterial spasm.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
Shepherd™ Peripheral Guidewires
Indications Statement: The Shepherd Peripheral guidewires are intended to facilitate the placement and exchange of balloon catheters or other interventional devices within the peripheral vasculature during Percutaneous Transluminal Angioplasty (PTA) or other intravascular interventional procedures.
Contraindications: The Shepherd Peripheral guidewires are not intended for use in the coronary or cerebral vasculatures or in patients judged not acceptable for percutaneous intervention.
Warnings: Contents supplied STERILE using an ethylene oxide (EO) process. Do not use if barrier is damaged. For single patient use only. Do not reuse, reprocess, or resterilize. Reuse, reprocessing, or resterilization may compromise the structural integrity of the device and/or lead to device failure, which, in turn, may result in patient injury, illness, or death. Reuse, reprocessing, or resterilization may also create a risk of contamination of the device and/or cause patient infection or cross-infection, including, but not limited to, the transmission of infectious disease(s) from one patient to another. Contamination of the device may lead to injury, illness, or death of the patient. Use the Guidewire prior to the “Use by” date on the package label, preceded by the symbol H . Refer to the instructions supplied with any interventional devices to be used in conjunction with the guidewire for their intended uses, contraindications, and potential complications. Do not manipulate the guidewire if resistance is met. Guidewire manipulations must always be observed under fluoroscopy. The Peripheral family of guidewires has distal ends of varying stiffness. Operate these guidewires carefully to minimize the risk of perforation or other damage to blood vessels. If the guidewire is removed and is to be re-inserted, it must be inspected for signs of damage (weakened or kinked segments) prior to re-introduction. Do not re-introduce if the guidewire is weakened or kinked.
Precautions:
This device should be used only by physicians trained in percutaneous, intravascular techniques and/or procedures. Carefully read all instructions prior to use. Observe all warnings and precautions noted throughout these instructions. Failure to do so may compromise guidewire performance and result in complications. Prior to use, confirm compatibility of guidewire outer diameter with the diagnostic or therapeutic device. Inspect guidewire prior to use for any surface irregularities, bends or kinks. Damaged and/or irregular guidewires should not be used. To avoid guidewire damage, do not withdraw the wire through a metal needle cannula. The tip section of the guidewire has a proper orientation for shaping. Identify the flexing plane before shaping. Shape in the same plane as that for flexure. Caution: Federal law (USA) restricts this device to sale by or on order of a physician.
Zilient® Peripheral Guidewires
Indications Statement: The Zilient® Peripheral guidewires are intended to facilitate the placement and exchange of balloon catheters or other interventional devices within the peripheral vasculature during Percutaneous Transluminal Angioplasty (PTA) or other intravascular interventional procedures.
Contraindications: The Peripheral guidewires are not intended for use in the coronary or cerebral vasculatures or in patients judged not acceptable for percutaneous intervention.
Prior to use, please see the complete Indications, Contraindications, Warnings, Precautions and Instructions for Use that can be found in the product labeling supplied with each device.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
Twister Core Wire Technology is a registered trademark of Lake Region Medical. CSI® and Zilient® are trademarks of Cardiovascular Systems, Inc. This product is distributed by CSI.
ViperCath™ XC Peripheral Exchange Catheter
Indications Statement: The ViperCath™ XC Peripheral Exchange Catheter is intended to guide and support a guide wire during access of the vasculature, allow for wire exchanges, and provide a conduit for the delivery of saline or diagnostic contrast agents.
Contraindications: Use of the ViperCath™ XC Peripheral Exchange Catheter is contraindicated in coronary or cerebral arteries, pregnant women, and patients with a contraindication to antiplatelet and/or anticoagulant therapy.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
Stealth 360® Peripheral Orbital Atherectomy System
Indications Statement: The Stealth 360® Peripheral Orbital Atherectomy System is a percutaneous orbital atherectomy system indicated for use as therapy in patients with occlusive atherosclerotic disease in peripheral arteries and who are acceptable candidates for percutaneous transluminal atherectomy. The OAS supports removal of stenotic material from artificial arteriovenous dialysis fistulae (AV shunt). The system is a percutaneous orbital atherectomy system indicated as a therapy in patients with occluded hemodialysis grafts who are acceptable candidates for percutaneous transluminal angioplasty.
Contraindications: The Systems are contraindicated for use in coronary arteries, bypass grafts, stents, or where thrombus or dissections are present.
Adverse Events: Although the incidence of adverse events is rare, potential events that can occur with atherectomy include: pain, hypotension, CVA/TIA, death, dissection, perforation, distal embolization, thrombus formation, hematuria, abrupt or acute vessel closure, or arterial spasm.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
ViperWire Advance® and ViperWire Advance® with Flex Tip Peripheral Guide Wires
Indications Statement: The Diamondback 360® and Stealth 360® Peripheral Orbital Atherectomy Systems are percutaneous orbital atherectomy systems indicated for use as therapy in patients with occlusive atherosclerotic disease in peripheral arteries and who are acceptable candidates for percutaneous transluminal atherectomy. The OAS supports removal of stenotic material from artificial arteriovenous dialysis fistulae (AV shunt). The system is a percutaneous orbital atherectomy system indicated as a therapy in patients with occluded hemodialysis grafts who are acceptable candidates for percutaneous transluminal angioplasty.
Contraindications: The Systems are contraindicated for use in coronary arteries, bypass grafts, stents, or where thrombus or dissections are present.
Adverse Events: Although the incidence of adverse events is rare, potential events that can occur with atherectomy include: pain, hypotension, CVA/TIA, death, dissection, perforation, distal embolization, thrombus formation, hematuria, abrupt or acute vessel closure, or arterial spasm.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
ViperCross™ Support Catheter
ViperCross peripheral support catheters are intended to be used in conjunction with steerable guidewires to access discrete regions of the peripheral vasculature. They may be used to facilitate placement and exchange of guidewires and other interventional devices and to sub-selectively infuse/deliver diagnostic and therapeutic agents.
Caution: Federal law (USA) restricts this device to sale by, or on the order of, a physician.
Indications
.014”
The ViperCross support catheter is intended to be used in conjunction with steerable guidewires to access discrete regions of the peripheral and/or coronary vasculature. It may be used to facilitate placement and exchange of guidewires and other interventional devices and to sub-selectively infuse/deliver diagnostic and therapeutic agents.
.018” & .035”
The ViperCross support catheter is intended to be used in conjunction with steerable guidewires to access discrete regions of the peripheral vasculature. It may be used to facilitate placement and exchange of guidewires and other interventional devices and to sub-selectively infuse/deliver diagnostic and therapeutic agents.
Contraindications (.014, .018, and .035)
The ViperCross support catheter is contraindicated for high pressure injections and for use in the cerebral vasculature.
Sapphire® II PRO Balloon Dilatation Catheter
The Sapphire® II PRO Balloon Dilatation Catheter is available in rapid exchange and over the wire catheters with a working length of 140 cm designed for both coronary and peripheral indications.
Indications Statement: The Sapphire II PRO Balloon Dilatation Catheter (1.0-4.0 mm configurations for the rapid exchange catheter and 1.0 mm for the over the wire configuration) is indicated for balloon pre-dilatation of a stenotic portion of a coronary artery or bypass graft stenosis (≥70% stenosis) for the purpose of improving myocardial perfusion. The Sapphire II PRO Balloon Dilatation Catheter (1.5-4.0 mm configurations) is indicated for balloon dilatation of the stenotic portion of a coronary artery or bypass, and graft stenosis in patients evidencing coronary ischemia for the purpose of improving myocardial perfusion. The catheter is indicated for balloon dilatation of a coronary artery occlusion for the treatment of acute myocardial infarction. The Sapphire II PRO Balloon Dilatation Catheter is also indicated for percutaneous transluminal angioplasty in the peripheral vasculature, including renal, femoral, popliteal, infra-popliteal, tibial, and peroneal arteries.
Contraindications: The use of the Sapphire II PRO Balloon Dilatation Catheter is contraindicated for use in patients with an unprotected left main coronary artery, for use in patients with coronary artery spasm in the absence of a significant stenosis, for use in the neuro vasculature, and where there is the inability to cross the target lesion with a guidewire.
Warnings: When using this type of device, the following warnings should be observed. This device is intended for single use only. Do not resterilize and/or reuse, as this can potentially result in compromised device performance and increased risk of cross-contamination. The safety and effectiveness of this balloon catheter for the treatment of in stent restenosis (ISR) has not been established. This balloon is not intended for the expansion or delivery of a stent. To reduce the potential for vessel damage, the inflated diameter of the balloon should approximate the diameter of the vessel just proximal and distal to the stenosis. PTCA in patients who are not acceptable candidates for coronary artery bypass graft surgery require careful consideration, including possible hemodynamic support during PTCA, as treatment of this patient population carries special risk. When the catheter is exposed to the vascular system, it should be manipulated while under high-quality fluoroscopic observation. Do not advance or retract the catheter unless the balloon is fully deflated under vacuum. If resistance is met during manipulation, determine the cause of the resistance before proceeding. Applying excessive force to the catheter can result in separation of the tip or balloon. Balloon pressure should not exceed the rated burst pressure (RBP) indicated on the package. The rated burst pressure is based on the results of in vitro testing. At least 99.9% of the balloons, (with a 95% confidence) will not burst at or below their rated burst pressure. Use of a pressure monitoring device is recommended to prevent over pressurization. To reduce the potential for air embolus into the vessel, use only the recommended balloon inflation medium. Never use air or any gaseous medium to inflate the balloon. Do not re-straighten a kinked hypotube; straightening a kinked metal shaft may result in breakage of the shaft. PTCA should only be performed at hospitals where emergency coronary artery bypass graft surgery can be quickly performed in the event of a potentially injurious or life-threatening complication.
Precautions: Use the catheter prior to the “Use By” date specified on the package. Prior to angioplasty, the catheter should be examined to verify functionality and ensure that its size and shape are suitable for the specific procedure for which it is to be used. The catheter system should be used only by physicians trained in percutaneous transluminal coronary or peripheral angioplasty. During the procedure, appropriate anticoagulant and vasodilator therapy must be provided to the patient as needed. After the procedure, anticoagulant therapy should be continued for a period of time as determined by the physician. Do not reinsert the catheter into the coil dispenser after procedural use. Discard all disposable devices used during this procedure per local requirements for medical device waste disposal. Do not use oil-based contrast medium, organic solvents or alcohols; there is a possibility of catheter leak, damage, or lubrication loss. The balloon deflation time has been established as 15 seconds based on in vitro bench testing results. Use with caution for procedures involving calcified lesions due to the abrasive nature of these lesions.
Adverse Events: Adverse effects due to the use of this product include, but are not limited to, the following: acute myocardial infarction, acute or subacute thrombosis, acute vessel closure, allergic reaction to device, contrast medium, or medication, aneurysm, arrhythmias, including ventricular fibrillation, arteriovenous fistula, coronary artery spasm, death, dissection (perforation, rupture, or injury) of the vessel, hemorrhage or hematoma, hypertension, hypotension, infection, restenosis of the dilated vessel, stroke, air embolism and embolization of fragmentation of thrombotic or athlerosclerotic material, total occlusion of the artery or bypass graft, unstable angina.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician. This product is distributed by CSI.
Manufactured By

Sapphire® NC24 and Sapphire® NC Plus Coronary Dilatation Catheters
The Sapphire NC24 and Sapphire NC Plus Coronary Dilatation Catheters are a percutaneous transluminal coronary angioplasty (PTCA) balloon catheter with a working length of 140 cm.
Indications Statement: The Sapphire NC24 and Sapphire NC Plus Coronary Dilatation Catheters are indicated for balloon dilatation of the stenotic portion of a coronary artery or bypass graft stenosis in patients evidencing coronary ischemia for the purpose of improving myocardial perfusion, balloon dilatation of a coronary artery occlusion for the treatment of acute myocardial infarction, in-stent restenosis and post-delivery expansion of balloon expandable coronary stents.
Contraindications: The use of Sapphire NC24 and Sapphire NC Plus Coronary Dilatation Catheters are contraindicated in the following patient types: patients with an unprotected left main coronary artery, and patients with coronary artery spasm in the absence of a significant stenosis.
Warnings: When using this type of device, the following warnings should be observed: this device is intended for single use only. Do not resterilize and/or reuse, as this can potentially result in compromised device performance and increased risk of cross-contamination. To reduce the potential for vessel damage, the inflated diameter of the balloon should approximate the diameter of the vessel just proximal and distal to the stenosis. PTCA in patients who are not acceptable candidates for coronary artery bypass graft surgery require careful consideration, including possible hemodynamic support during PTCA, as treatment of this patient population carries special risk. When the catheter is exposed to the vascular system, it should be manipulated while under high-quality fluoroscopic observation. Do not advance or retract the catheter unless the balloon is fully deflated under vacuum. If resistance is met during manipulation, determine the cause of the resistance before proceeding. Balloon pressure should not exceed the rated burst pressure (RBP) indicated on the package. The rated burst pressure is based on the results of in vitro testing. At least 99.9% of the balloons, (with a 95% confidence) will not burst at or below their rated burst pressure. Use of a pressure monitoring device is recommended to prevent over pressurization. To reduce the potential for air embolus into the vessel, use only the recommended balloon inflation medium. Never use air or any gaseous medium to inflate the balloon. Do not re-straighten a kinked hypotube; straightening a kinked metal shaft may result in breakage of the shaft. PTCA should only be performed at hospitals where emergency coronary artery bypass graft surgery can be quickly performed in the event of a potentially injurious or life-threatening complication.
Precautions: Use the catheter prior to the “Use By” date specified on the package. Prior to angioplasty, the catheter should be examined to verify functionality and ensure that its size and shape are suitable for the specific procedure for which it is to be used. The catheter system should be used only by physicians trained in percutaneous transluminal coronary angioplasty. During the procedure, appropriate anticoagulant and coronary vasodilator therapy must be provided to the patient as needed. After the procedure, anticoagulant therapy should be continued for a period of time as determined by the physician. Do not reinsert the PTCA catheter into the coil dispenser after procedural use. Discard all disposable devices used during this procedure per local requirements for medical device waste disposal. Do not use oil-based contrast medium, organic solvents or alcohols; there is a possibility of catheter leak, damage or lubrication loss. The balloon deflation time has been established as 15 seconds based on in vitro bench testing results.
Adverse Events: Adverse effects due to the use of this product include, but are not limited to, the following: acute myocardial infarction, acute vessel closure, arrhythmias, including ventricular fibrillation, arteriovenous fistula, coronary artery spasm, coronary vessel dissection, perforation, rupture, or injury, death, drug reactions, allergic reaction to contrast medium, hemorrhage or hematoma, hypertension, hypotension, infection, restenosis of the dilated vessel, stroke, air embolism and embolization of fragmentation of thrombotic or athlerosclerotic material, total occlusion of the coronary artery or bypass graft, unstable angina.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician. This product is distributed by CSI.
Manufactured By

Teleport® Microcatheter
The Teleport® family of microcatheters are single lumen catheters, offered in two shaft sizes (2.0 F and 2.1 F) with working lengths of 135 cm or 150 cm, designed for use in the coronary, peripheral, and abdominal vasculature.
Indications Statement: The Teleport microcatheters are indicated for supporting and facilitating the placement of guidewires in the coronary and peripheral vasculature, exchanging guidewires in the coronary and peripheral vasculature, and the delivery of contrast media into the coronary, peripheral, and abdominal vasculature.
Contraindications: The use of the Teleport microcatheters are contraindicated for use in patients with an unprotected left main coronary artery, for use in patients with arterial spasm in the absence of significant stenosis, and for use in the neurovasculature.
Warnings: When using this type of device, the following warnings should be observed. This device is intended for single use only. Do not resterilize and/or reuse, as this can potentially result in compromised device performance and increased risk of cross-contamination. Do not modify this product for any reason. Modification of this product may compromise the integrity and performance of the product. Use of a modified product may result in vascular injury. The patient may suffer from subacute thrombosis, vascular complications, or bleeding complications by using this microcatheter. Therefore, it should be well examined if the intervention procedure will be applicable for the patient. The device must always be operated under high-resolution fluoroscopic guidance. Particular attention should be paid when inserting or withdrawing the device into stenotic areas, highly calcified lesions, stent struts, and narrower vessels than the product. Abrasion may result in damage or rupture of the device and the hydrophilic coating which may cause vascular injury, perforation, or distal emboli. If abnormal resistance is detected during use of this product, do not continue the operation, avoid excessive manipulations, and carefully remove the entire catheter system while paying full attention to avoid complications. Continuing the operation while the cause of the problem is not identified may cause damage to, or rupture of, the catheter, and damage the blood vessel. This microcatheter is coated with hydrophilic coating. Therefore, the microcatheter is highly lubricious. Always confirm the position of the distal end of the microcatheter by fluoroscopy and manipulate the microcatheter carefully. Do not insert the guidewire by force or advance it rapidly when the microcatheter is bent or twisted. Such manipulations may cause rupture or damage of the microcatheter, or perforation of the blood vessel. Always advance the guidewire ahead of the microcatheter before attempting any manipulation of the microcatheter. If the guidewire is not advanced ahead of the microcatheter, the blood vessel may be damaged or perforated, or the microcatheter may be damaged. Always hold the proximal hub with one hand and turn the catheter carefully while regularly releasing the accumulated torsion of the catheter. Never turn the catheter continuously while holding the proximal hub with both hands or use any other means to apply force. When releasing the accumulated torsion, be sure to open the hemostatic valve on the Y-connector. Do not turn the catheter in the same direction, either clockwise or counterclockwise, for more than 20 consecutive turns. If resistance is felt while turning the catheter, do not proceed with further rotation even if the 20-turn limit has not been reached. Identify the cause of resistance under fluoroscopy, and take appropriate action. Never continue the operation without identifying the cause; continuing rotation may damage or rupture the catheter or damage the blood vessel.a lways advance the guidewire ahead of the microcatheter before attempting any manipulation of the microcatheter. If the guidewire is not advanced ahead of the microcatheter, the blood vessel may be damaged or perforated, or the microcatheter may be damaged. Always hold the proximal hub with one hand and turn the catheter carefully while regularly releasing the accumulated torsion of the catheter. Never turn the catheter continuously while holding the proximal hub with both hands or use any other means to apply force. When releasing the accumulated torsion, be sure to open the hemostatic valve on the Y-connector. Do not turn the catheter in the same direction, either clockwise or counterclockwise, for more than 20 consecutive turns. If resistance is felt while turning the catheter, do not proceed with further rotation even if the 20-turn limit has not been reached. Identify the cause of resistance under fluoroscopy, and take appropriate action. Never continue the operation without identifying the cause; continuing rotation may damage or rupture the catheter or damage the blood vessel. When infusing contrast media, the device must be operated under high-resolution fluoroscopic guidance, confirming that the contrast media is being infused from the tip of the device. If the contrast media is not being infused, infusion must be stopped and the device must be replaced. If the device lumen is occluded, the device may be dilated, damaged, or ruptured, resulting in a life-threatening adverse event due to spurting contrast media. Injection pressure must not exceed 300 psi (the maximum injection pressure) when injecting contrast media using a power injector. Exceeding the maximum injection pressure may cause damage to the microcatheter. Discontinue injection if irregular resistance is felt at the syringe. The microcatheter may be bent or blocked. Excessive pressure may cause expansion and/or rupture of the microcatheter. Do not use a power injector to infuse contrast media when the microcatheter is bent or occluded. It may cause damage to the microcatheter such as expansion or breakage.
Do not use guidewires larger than the recommended size. Resistance may be felt while advancing or withdrawing a guidewire larger than the recommended size, which may cause the catheter to become damaged or break, or the blood vessel to become damaged. If the device is inserted into vessels and the guidewire is to be replaced, insert the guidewire carefully. If any resistance is encountered, the operation must be discontinued immediately, and the device and the guidewire(s) withdrawn together. The device may be damaged and the tip may be cut. Do not wipe the surface of the microcatheter with gauze or absorbent cotton soaked with alcohols, gluconic acid chlorhexidine aqueous solution, or similar solutions as it may significantly deteriorate the lubricity of the microcatheter. Repeated insertion and withdrawal of the device may lead to deterioration of the hydrophilic coating. Continuous use of the device with deteriorated hydrophilic coating may cause vascular damage. This may also increase the risk of the tip being trapped, damaged, or ruptured.
Precautions: Use the microcatheter prior to the “Use By” date specified on the package.
Prior to use, the catheter should be examined to verify functionality and ensure that its size and length are suitable for the specific procedure for which it is to be used. Check to ensure that the microcatheter was not damaged during transportation. Do not use if the package and/or the product is suspected to be damaged. This product must be used under fluoroscopy by a physician who is fully trained in interventional procedures. Check the patient condition before the procedure. Provide appropriate anticoagulant therapy if necessary. When inserting the guidewire into the microcatheter that is already placed in the blood vessel, carefully operate the guidewire not to damage the microcatheter at the bend sections. When using a guiding catheter fitted with a stopcock, do not manipulate the stopcock after inserting the microcatheter into the guiding catheter. The microcatheter may be damaged if the stopcock is manipulated during the insertion. Operate the microcatheter carefully to avoid damage, kinks, or bends, especially when inserting into the guiding catheter. Flush the surface and the lumen of the microcatheter continuously with sterile heparinized saline during its use to maintain lubricity. When inserting or exchanging the microcatheter, flush the lumen of the guiding catheter and the microcatheter system continuously with sterile heparinized saline. This product is not intended for drug infusion other than contrast media. This product has not been designed for drug infusion other than contrast media and its safety has not been established for this indication. When infusing contrast media, read the Instructions for Use provided with such contrast media and comply with instructions, precautions, and warnings. Use the extension tube when contrast media is injected by using power injector. Confirm that the inserted microcatheter does not have a kink, knot, torsion, or occlusion before injecting contrast media. Flush the lumen of the microcatheter sufficiently with sterile heparinized saline especially after injecting contrast media. Discard all disposable devices used during this procedure per local requirements for medical device waste disposal.
Adverse Events: Adverse effects due to the use of this product include, but are not limited to, the following: acute myocardial infarction, acute, or subacute, thrombosis, acute vessel closure, allergic reaction to device, contrast medium, or medication, aneurysm, arrhythmias, arteriovenous fistula, death, dissection (perforation, rupture, or injury) of the vessel, distal emboli, hemorrhage or hematoma, hypertension, hypotension, infection, ischemia, stroke by air embolism or embolization of fragments of thrombotic or athlerosclerotic material, total occlusion of the artery resulting in ischemia, vascular spasm.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician. This product is distributed by CSI.
Manufactured By

WIRION® Peripheral Embolic Protection System
Indications Statement: WIRION® is indicated for use as an embolic protection system to contain and remove embolic material (thrombus/debris) while performing atherectomy in calcified lesions of the lower extremities. The diameter of the vessel at the site of filter basket placement should be between 3.5mm to 6.0mm. WIRION may be used with commercially available 0.014″ guide wires.
Contraindications: The system is contraindicated for patients with severe allergy to Heparin, patients with uncorrected bleeding disorder, and patients in whom anticoagulant and antiplatelet therapy is contraindicated.
Caution: Federal law (USA) restricts this device to sale by, or on the order of, a physician.
JADE® PTA Balloon Catheter
The Jade PTA Balloon Dilatation Catheter is indicated for Percutaneous Transluminal Angioplasty in the peripheral vasculature, including iliac, femoral, ilio-femoral, popliteal, infra-popliteal, and renal arteries, and for the treatment of obstructive lesions of native or synthetic arteriovenous dialysis fistulae. This device is also indicated for post-dilation of balloon expandable and self-expanding stents in the peripheral vasculature. Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
This product is distributed by CSI.
Manufactured By

Scoreflex® NC Scoring Balloon
INDICATIONS: The Scoreflex NC Scoring PTCA Catheter is indicated for: Balloon dilatation of a de novo stenotic portion of a coronary artery and in-stent restenosis in coronary arteries in patients evidencing coronary ischemia for the purpose of improving myocardial perfusion.
CONTRAINDICATIONS:I The use of the Scoreflex NC Scoring PTCA Catheter is contraindicated in the following patient types: Patients with an unprotected left main coronary artery., Patients with coronary artery spasm in the absence of a significant stenosis.
WARNINGS: When using this type of device, the following warnings should be observed: This device is intended for single use only. Do not resterilize and/or reuse, as this can potentially result in compromised device performance and increased risk of cross-contamination. This balloon is not intended for the expansion or delivery of a stent. PTCA in patients who are not acceptable candidates for coronary artery bypass graft surgery require careful consideration, including possible hemodynamic support during PTCA, as treatment of this patient population carries special risk. To reduce the potential for vessel damage, the inflated diameter of the balloon should approximate the diameter of the vessel just proximal and distal to the stenosis.When the catheter is exposed to the vascular system, it should be manipulated while under high-quality fluoroscopic observation. Do not advance or retract the catheter unless the balloon is fully deflated under vacuum. If resistance is met during manipulation, determine the cause of the resistance before proceeding. Applying excessive force to the catheter can result in tip or catheter breakage, catheter kink, or balloon separation.Do not twist the catheter shaft in excess of 180 degrees when the tip is constrained. Balloon pressure should not exceed the rated burst pressure (RBP) indicated on the package. The rated burst pressure is based on the results of in vitro testing. At least 99.9 percent of the balloons, (with a 95 percent confidence) will not burst at or below their rated burst pressure. Use of a pressure monitoring device is recommended to prevent over pressurization.To reduce the potential for air embolus into the vessel, use only the recommended balloon inflation medium. Never use air or any gaseous medium to inflate the balloon. Do not re-
straighten a kinked hypotube; straightening a kinked metal shaft may result in breakage of the shaft. PTCA should only be performed at hospitals where emergency coronary artery bypass graft surgery can be quickly performed in the event of a potentially injurious or life-threatening complication
PRECAUTIONS: Use the catheter prior to the “Use By” date specified on the package. Prior to angioplasty, the catheter should be examined to verify functionality and ensure that its size and shape are suitable for the specific procedure for which it is to be used.The catheter system should be used only by physicians trained in percutaneous transluminal coronary angioplasty. During the procedure, appropriate anticoagulant and coronary vasodilator therapy must be provided to the patient as needed. After the procedure, anticoagulant therapy should be continued for a period of time as determined by the physician. Never advance the Scoreflex NC Scoring PTCA Catheter without the guidewire extending from the tip. Do not use oil-based contrast medium, organic solvents, or alcohols; there is a possibility of catheter leak, damage, or lubrication loss. The balloon deflation time has been established as 15 seconds based on in vitro bench testing results. Do not reinsert the PTCA catheter into the coil dispenser after procedural use. Discard all disposable devices used during this procedure per local requirements for medical device waste disposal.
Caution: Federal law (USA) restricts this device to the sale by or on the order of a physician.
See the instructions for use before performing Scoreflex NC Scoring PTCA Catheter procedures for detailed information regarding the procedure, indications, contraindications, warnings, precautions, and potential adverse events.
This product is distributed by CSI.
Manufactured By

JADE, Sapphire, Scoreflex, Teleport, OrbusNeich are registered trademarks of OrbusNeich Medical Company Limited. All other trademarks cited herein are owned by their respective owners.
G-70-1092 Rev03.