Treat with Confidence
Be Calculated® with CSI’s portfolio of innovative tools
Be Calculated® with CSI’s portfolio of innovative tools
CSI also offers a growing portfolio of innovative tools to help physicians have a calculated approach to PCI.
CSI’s innovative and evidence-based solutions perform in complex calcium so physicians can treat with confidence. More than 100,000 patients have been treated with the Diamondback 360® Orbital Atherectomy System. Now enrolling for the ECLIPSE clinical trial. 10, 51-53
Uniquely designed for calcium: Enables simultaneous modification of both intimal and medial calcium for optimal stent delivery, expansion and apposition in severely calcified lesions. One device treats eccentric, concentric and nodular calcium1-5.

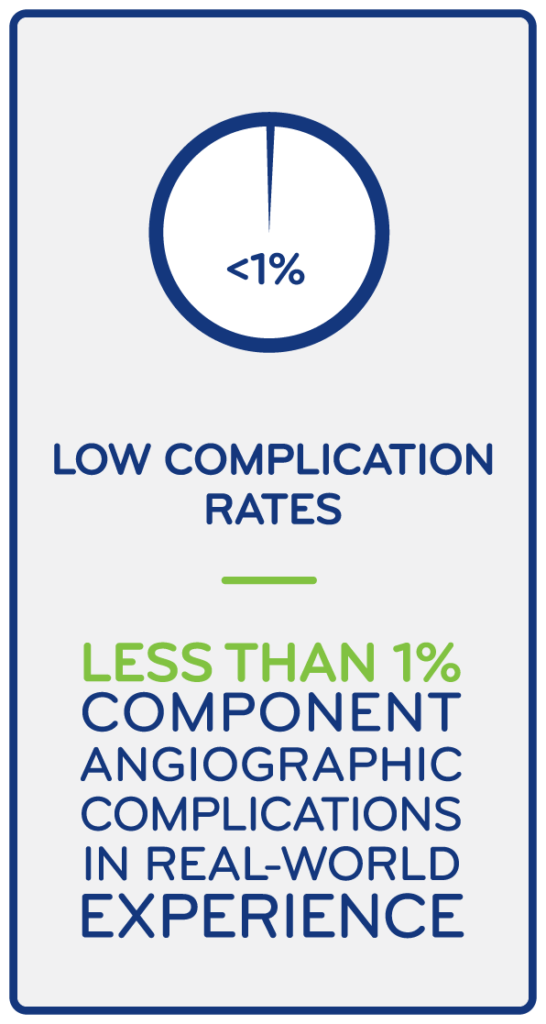


Preceptor/Proctorships
Nurse/Technologist
Programs
Regional & National
Courses

Intermediate: An
Introduction to Therapy Technology.
Taught by Certified Instructors
Advanced: Expanding Skills and Techniques. Best Practices
Masters: Leading Experts.
Clinical Updates. Latest
Techniques
OAS Certification
Fellows Course &
Milestones Calendar
Online Learning Modules
& Focused Webinars
OAS Certification
Case Library
CSIQ Learning Management
System
Peer-to-Peer Webinars
Live Case Broadcasts
Join our email list to get updates about our commitment to constant progress. You’ll see how CSI and orbital first users are shaping the future of CSI.
You can unsubscribe from these communications at any time. Please review our Privacy Policy and Terms of Use for more information.
If you believe someone may be unintentionally or intentionally violating the law or the principles or standards, report the known or suspected violations by visiting our compliance page.
CSI is a registered trademark of Cardiovascular Systems, Inc. ©2023 All Rights Reserved.